
On February 9, 2025, the last three EU member states should have introduced the European pharmaceutical serialization system. The European Falsified Medicines Directive (EU-FMD) came into force on February 9, 2019. However, Italy, Belgium, and Greece were granted a six-year exemption, as these countries already had existing systems for verifying and tracing medicinal products.
In Italy, the authenticity of medicines and the identification of individual packs have been guaranteed by the “Bollino system”. Unlike in the rest of the EU, packaging labels, the so-called Bollino stickers, and serial numbers are issued by the state. Another unique feature is that track and trace data is linked to health information. As example information about drug safety and reimbursement for medicinal products.
How can we assist you, Italy?
Schedule a non-binding demo with us!
Do you need assistance with implementing the EU-FMD? We provide expert guidance, intuitive software, and a flexible pricing model. Let’s work together to ensure your compliance.
The “Bollino system”
The Bollino ID system was introduced on February 1, 2002, for prescription and OTC medicines. Bollino is an adhesive label (see image). Manufacturers purchase it from the Italian Government Printing Office (IPZS) and apply it to the drug packaging. It displays important medicine data in words, two barcodes, and a data matrix.
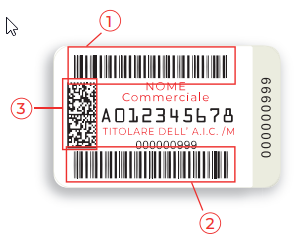
The upper code (1) contains the A.I.C. code, an authorization number with nine digits, which is issued by the Italian Medicines Agency (A.I.F.A.). A serial number (UID) for unique identification of the pack is in the lower barcode (2). Also, it is requested from the Italian Government Printing Office (IPZS). Finally, the data matrix summarises both code information, the A.I.C. code, and the UID.
A central database was introduced under Article 40 of Law No. 39 of March 1, 2002. It enables the authorities to track every pharmaceutical package along the supply chain. The database is part of the Italian National Health Information System (NHIS). It allows to monitor all information relating to an individual medicinal product. Every incident, be it a counterfeit drug or a side effect, can thus be traced back directly to a specific medicine. Because of that necessary treatment measures can be initiated immediately.
Upcoming changes introducing the EU-FMD in Italy
The introduction of the EU-FMD in Italy will bring some advantages as well as changes. Therefore, it is important to start preparing at an early stage. Depending on the number of products and the size of the company, enough time and money must be planned for artwork, labeling, and the conversion of production lines to the GS1 standard. Manufacturers who already supply other European countries have a clear advantage here. They can use their own print templates, while other companies have previously received the Bollino stickers from the Italian government.
Another issue is the exchange of information with the EU hub. Instead of the A.I.C. code and the UID, provided by the Italian government, packaging units are labeled with a Global Trade Item Number (GTIN), batch number, the expiry date of the medicinal product, and a randomly generated serial number. The latter, like the artwork, can be generated by the manufacturers themselves. The GTIN, on the other hand, is applied once from GS1 and is valid worldwide. This data is usually transmitted to the EU hub via a serialization provider. The extent to which this is possible via the existing central database in Italy is still in discussion.
Timeline for the Italian “EU-FMD” project
Furthermore, it remains to be seen how OTC drugs that are not subject to serialization under the EU-FMD will be handled in the future and what will happen to the connection to the NIHS. Experts assume that the result will be a combination of the new Italian National Medicines Verification System (NMVS) and the existing infrastructure, including a link to the NHIS.
On 06.02.2025, the Italian Council of Ministers published a decree making a 2-year stabilization phase official. According to the decision, the transition phase will start on the actual date of the EU-FMD deadline, 09.02.2025. From this date, the Bollino and serialization according to the EU-FMD will be allowed in Italy and can therefore be used simultaneously. The deadline for using the Bollino is therefore 08.02.2027. On the following day, 09.02.2027, only serialization in accordance with the European regulation is permitted. Further information, such as the specifications of the Unique Identifier, the availability and connection of the Italian NMVO and the security features of the sales pack will be announced in the course of time.
How can tracekey help?
At tracekey, we have been supporting pharmaceutical companies with the implementation of serialization projects for more than 10 years. We know the challenges small and medium-sized companies in particular have to face when it comes to complex regulatory requirements. That´s because we experienced live the introduction of the EU-FMD. Many of our existing customers date back to this time. Our service is not limited to the one-off fulfillment of regulations. Thanks to our longstanding experience, we know that the conditions for serialization are constantly evolving. Be it the integration of new business partners or a change in production or regulations. Here, too, we are at your side as a reliable partner. We adapt our software accordingly and ensure your business processes are always optimally supported.
If this sounds interesting to you, feel free to contact us.
[Disclaimer] This information is only one possible interpretation of the regulations. They are also in a constant state of change, so the information in this article may be incomplete or out of date. The above article is expressly no legal advice. Please refer to the official documents for information before making any business decisions. (Status of information: February 2025)