The Single Registration Number (SRN) is the most important identifier for all economic operators working in EUDAMED. It serves for unique identification across Europe in the European Database on Medical Devices (EUDAMED). To apply for the SRN/Actor ID, economic operators must register in the Actor Module of EUDAMED.
The Single Registration Number (SRN) is essential for using EUDAMED, which is why it is also referred to as the EUDAMED Number or Actor Identifier, abbreviated as Actor ID. However, there is a difference between the SRN and the Actor ID: The unique identification number is called SRN when the actor is a manufacturer, authorized representative, or importer of MDR and/or IVDR products registered under MDR Article 31/IVDR Article 28. Otherwise, the identifier is called Actor ID.
How and where can you apply for the Single Registration Number?
The SRN is generated and assigned by EUDAMED as part of the registration process in the Actor Module. To be assigned an SRN/Actor ID, an economic operator, such as a medical device manufacturer, must initiate the registration process through the Actor Module of EUDAMED. This is the first of the six EUDAMED modules.
Who can apply for the SRN?
The Single Registration Number (SRN) can be applied for by all economic operators. These roles include:
- Manufacturer (MF)
- Authorized Representative (AR)
- System & Procedure Pack Producer (PR)
- Importer (IM)
The registration process in the Actor Module
- The economic operator submits a registration request in the Actor Module of EUDAMED.
- For non-EU actors, the request must be verified by the Authorized Representative and then forwarded to the national authority.
- Once the national authority approves the registration, it issues the SRN/Actor ID generated by EUDAMED.
- The economic operators receive the SRN/Actor ID via email from EUDAMED.
What is the Single Registration Number (SRN) used for?
The SRN is used to register in EUDAMED and serves as a unique identification for all economic operators interacting with EUDAMED. Each actor receives one SRN/Actor ID per role. For example, if a manufacturer also acts as an importer, they need an SRN or Actor ID for each role and must register separately for both roles in EUDAMED.
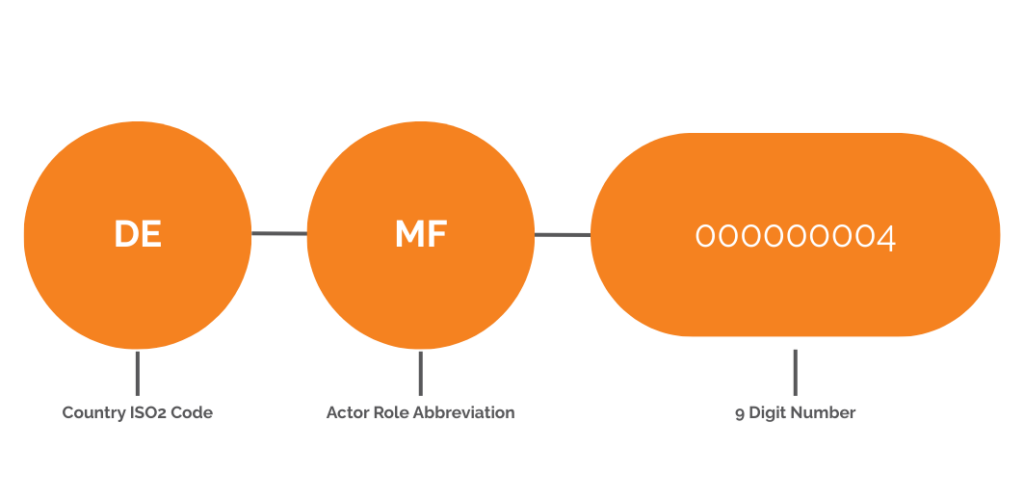
How is the SRN structured?
SRN and Actor ID have the same structure. They consist of three fixed parts:
- Country ISO2 code
- Actor Role Abbreviation, such as MF, AR, PR, or IM
- and 9 characters (numbers)
A code for a medical device manufacturer from Germany could look like this: DE-MF-000000004.
The SRN is also linked to all UDIs of an economic operator and serves for the unique assignment of information. Do you already know how you will store your UDIs in EUDAMED? As a third-party provider of a software solution for EUDAMED, we offer the perfect tool for UDI management. This significantly simplifies the upload of UDIs and the associated product information. Feel free to schedule a demo appointment or visit our website to learn more about the mytracekey UDI Manager.