Have you ever struggled to find the information you need in a leaflet? Perhaps you’ve even given up on using it because you couldn’t fold it back up correctly. A better solution would be to access the instructions for your medication on your smartphone. This would save you time and frustration. If you are familiar with these thoughts or are interested in the topic from a regulatory perspective, you have come to the right place. Our article is about electronic product information for medicines or ePI for short.
In January 2020, the European Medicines Agency (EMA), the European Medicines Agency (HMA), and the European Commission came together. They defined principles that should apply to the development of a digital package leaflet. Outside of Europe, there are several pilot projects on this topic with exciting approaches, too.
What is electronic product information (ePI)?
In Europe and most other countries, the provision of a package leaflet is required by law. It informs doctors, pharmacists, and patients about the safe use of a medicine. The electronic version of the product information pursues the same goal. In addition to the instructions for use, it contains a summary of product characteristics (SmPC) and the labeling requirements for the pharmaceutical packaging.
An ePI is usually a file in XML, JSON, or RDF format. Compared to other digital standards such as MS Word or PDF, data can be easily extracted from this format without losing its external structure. This simplifies the electronic exchange of information between authorities and manufacturers via web portals or digital platforms and enables digital post-processing.
What are the advantages?
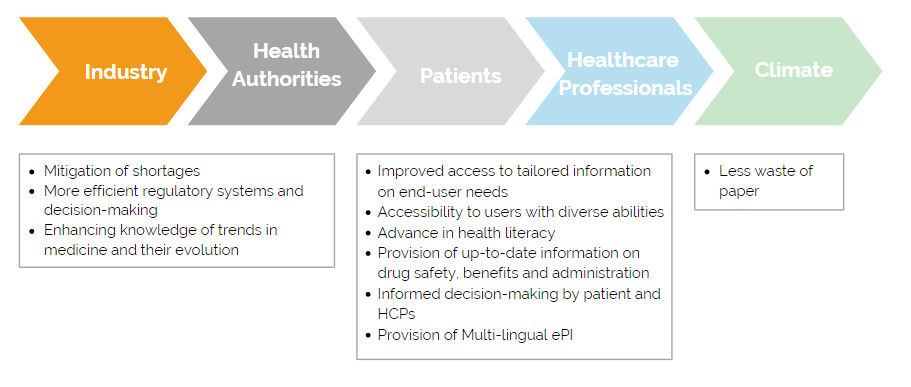
The coronavirus crisis has shown how important it is to work together digitally and make decisions in a short time. That also applies to the exchange of information between industry and authorities. Manufacturers can use ePIs to regularly forward reports on current therapeutic procedures, diseases, and their causes to the authorities. The latter could be informed more quickly by the industry about drug shortages. This way they can take more efficient measures, such as the redistribution of drugs to other EU member states.
With the aid of ePIs, patients and healthcare professionals will always have access to up-to-date information on the respective medicine. Regulatory changes to product information would not have to wait for the next printing of a new package leaflet. Furthermore, patients could read the package leaflet in their preferred language, regardless of the country they purchased the medicine in. Depending on the situation, a paper version of the package leaflet could be completely avoided in the future.
What are the challenges?
In addition to the obvious advantages, the development of ePIs brings with it some challenges. An open dialogue between all parties is important for coordinating such a big project. For example, when deciding on a standardized ePI format, sufficient funding and embedding the ePIs in existing national or international telematics structures.
One issue frequently discussed internationally and handled differently depending on the country is how patients are given access to the digital patient information leaflet. India proposes to make the product information available on the manufacturer’s website. In contrast, Taiwan plans to centralize data management through the TFDA. If the website link is embedded in a QR code, it can be scanned with any smartphone but takes up additional space on the pharmaceutical packaging. Therefore, many pharmaceutical representatives propose integrating the website link into the GS1 data matrix and developing a scanner app as part of an international project. In the United Arab Emirates, it has been decided to avoid the discussion altogether and introduce both codes as mandatory. For all other countries, GS1 recommends contacting local authorities and GS1 representatives to find out more and, if necessary, to influence the emerging regulations.