UDI Product Data Management for Medical Devices
Product Information Management (PIM) for the implementation of global medical device regulations
The Medical Device Regulation (MDR) is considered to be one of the most complex regulations worldwide and comes along with many obstacles and hurdles for manufacturers of medical devices. In addition to recertification (keyword “Notified Bodies”) and the increased scope of clinical studies, one of the most important components of the MDR is the collection of product data and subsequent UDI management. Some of these are publicly accessible via EUDAMED and are intended to ensure greater transparency and safety. Medical devices will become traceable through the Unique Device Identification (UDI), so that recalls can be carried out more quickly and in a more targeted manner, and product counterfeits can be better detected.
Medical technology companies need a validated system that is suitable for everyday use and which enables them to overcome the challenges of the MDR-UDI and other international UDI regulations. Customer groups, such as hospitals and GPOs, also ask about the quality and consistency of the transmitted product data. Efficient and secure UDI data management has therefore become an important topic for medical device producers.
Your Benefits
- Simple Product Templates
- Adaptive Support Models
- Manage Product Hierarchies
- Single Point of Truth
- Connectors to Authorities & Business Partners
- Redundant GxP Cloud Infrastructure
- Regular Software Updates
Challenges of the Medical Device Industry
- Product data is distributed within the company and across different partners
- Data must be prepared for different target markets
- Manual processes are a frequent source of errors
- Lack of clarity as to which attributes must be reported to EUDAMED for a specific product
- Standards for product data are different depending on regulations/stakeholders
Implement UDI Master Data Management with tracekey
Our mytracekey platform for medical devices supports the medical device industry in preparing and managing their product master data following regulatory requirements such as the EU MDR. Through our many years of experience in the life science industry, we have learned to adapt our software solutions to highly regulated industries. That’s why we take a close look at your requirements and developed software that helps you to overcome obstacles and challenges. To do this, we connect various authorities and systems, such as EUDAMED, with manufacturers, contract manufacturers, distributors, hospitals, and their respective business partners.
Your Advantages
- Manual processes and Excel sheets are replaced by a digital and unique storage location
- We look after you personally and at eye level right from the start
- Unnecessary product recalls due to incorrect or inconsistent data are prevented
- You manage your product data in an MDR-compliant manner from the outset
- Comprehensive UDI management
We enable our network of over 300 customers and business partners to manage product data, generate serial numbers, exchange data, and comply with global regulations – all centrally managed in one place. This gives you a Single Point of Truth (SPOT) where you can keep your product data always up-to-date and compliant.
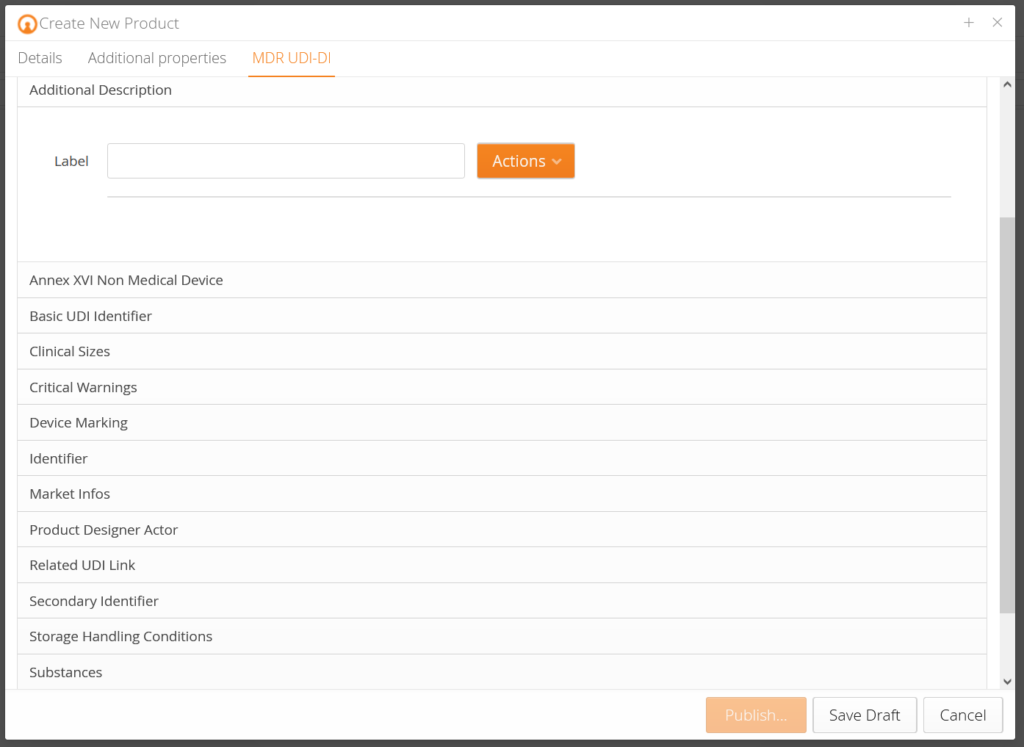
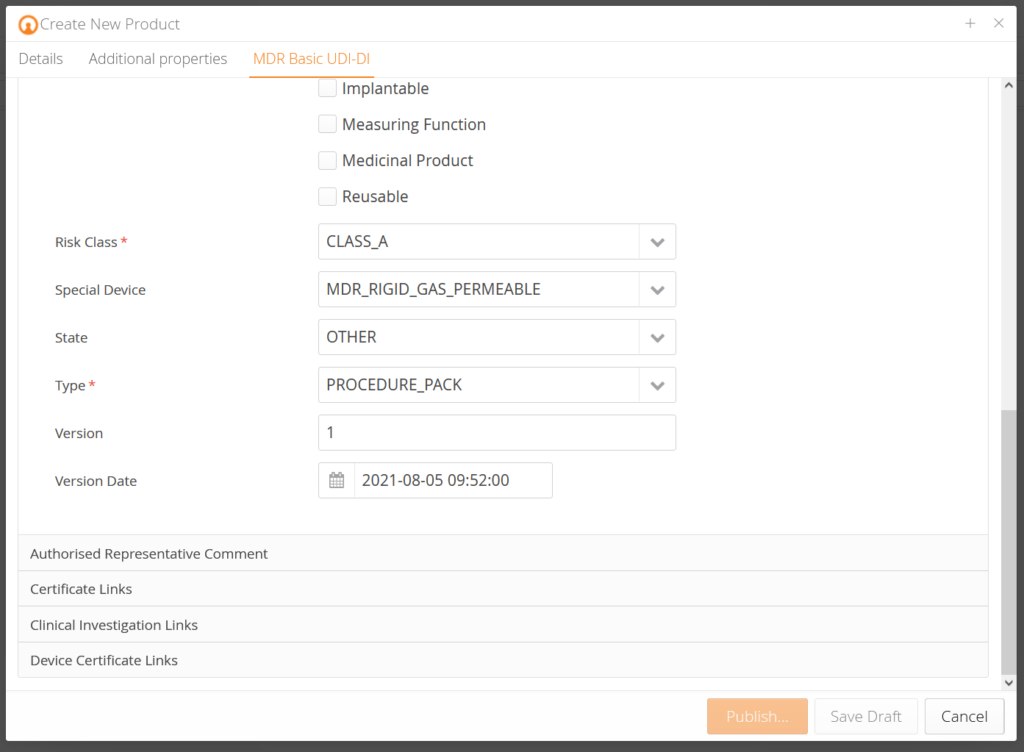
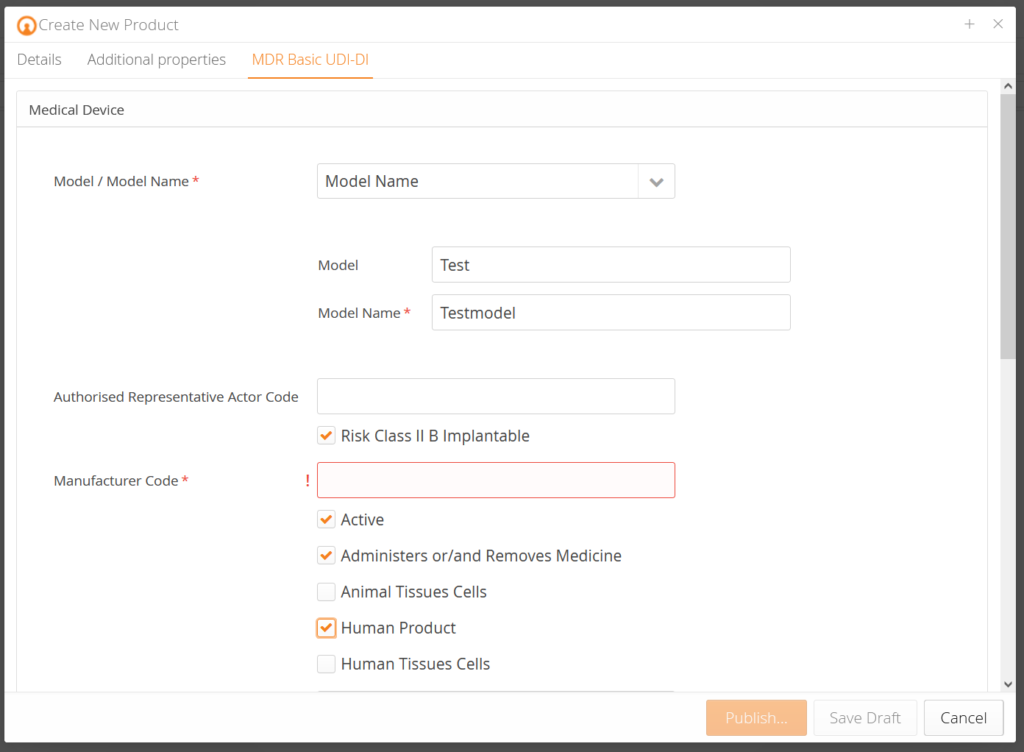
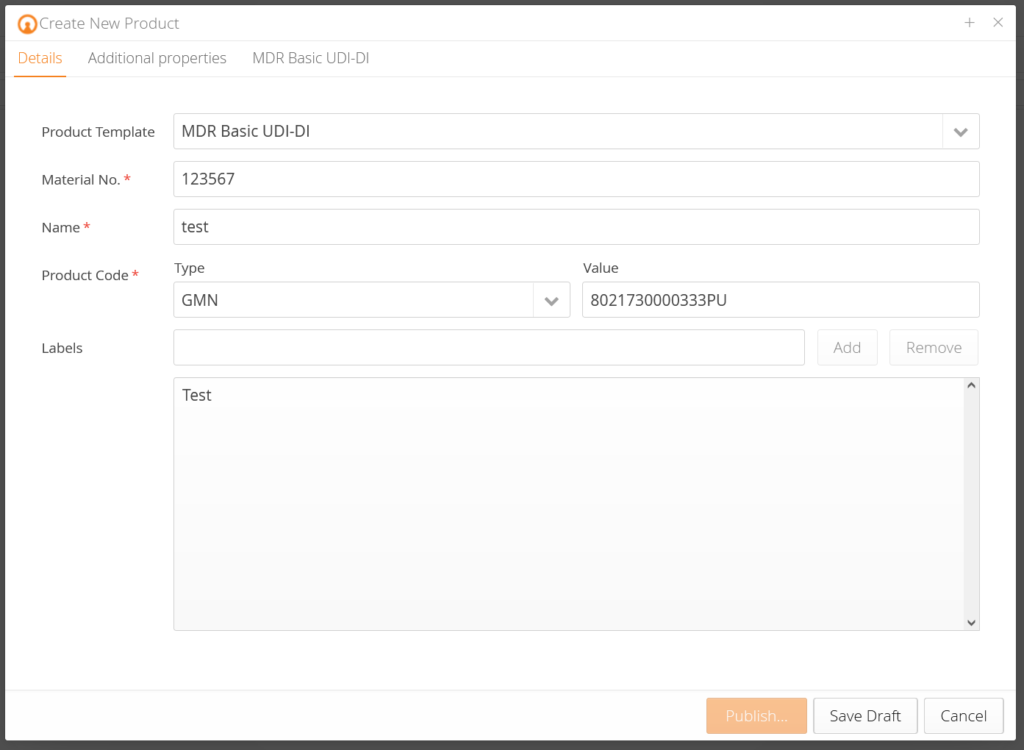
Our customers receive pre-designed data templates that query the corresponding product data for each product and pre-validate them according to EUDAMED requirements. In addition, there is an explanation for each data field so that you know which data is demanded. Based on the change history (audit trail), you know who changed what at which time. Traceability – made simple.
Addressing New Challenges Targeted:
Data quality requirements: Our solution pre-validates your data and maps product hierarchies.
Reporting to authorities: Reporting to authorities is part of our daily routine, whether, via automated interfaces or manual workflows, our system and our team know how to do it.
Integration of the supply chain: Our solution functions as a central system in which business partners can be connected as required so that nothing stands in the way of a clear and documented exchange of data.
International regulations: We know that a system is only sustainable if it can be flexibly adapted to future demands. That’s why we also keep an eye on international regulations.
Close to the customer: Our modular support offers are geared to the requirements of our customers. Regardless of the support model, you’ll choose, we always support our customers in the best possible way.
How is mytracekey MedTech Implemented?
The implementation time depends on the number of your products. To get started, you will receive intensive training to familiarise yourself with the new processes and the system itself. After the intensive training, you can assess where you are still missing product data. As a cloud system, our service is ready for immediate use.
We Support You in All Matters Relating to MDR/UDI

Download our White Paper on “7 steps to UDI compliance” here. Download Whitepaper MDR-UDI Compliance
-> Get an overview of the EUDAMED modules
-> Can you meet the MDR requirements with your ERP system?
-> We have compiled helpful information material on the MDR and IVDR for you