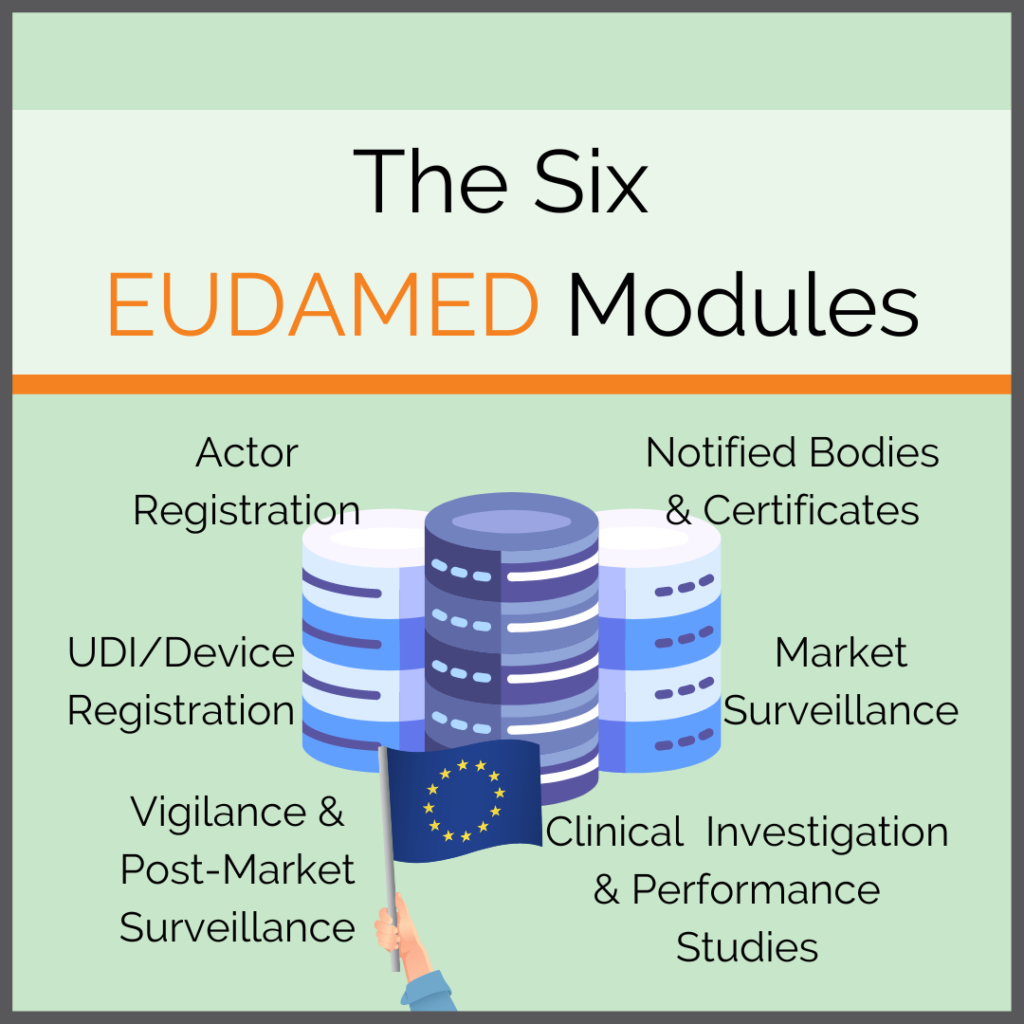
The European Commission regularly publishes documents to assist in the use of EUDAMED. Due to the complexity of EUDAMED as well as the multitude of instructional documents, it is nevertheless important to get an overview yourself. This way you will learn which aspects apply to your own company and your products. It is the only […]
7 Tips for Data Preparation in EUDAMED